Room Temperature Superconductor: Holy Grail or Red Herring?

Abigail Malate, Staff Illustrator
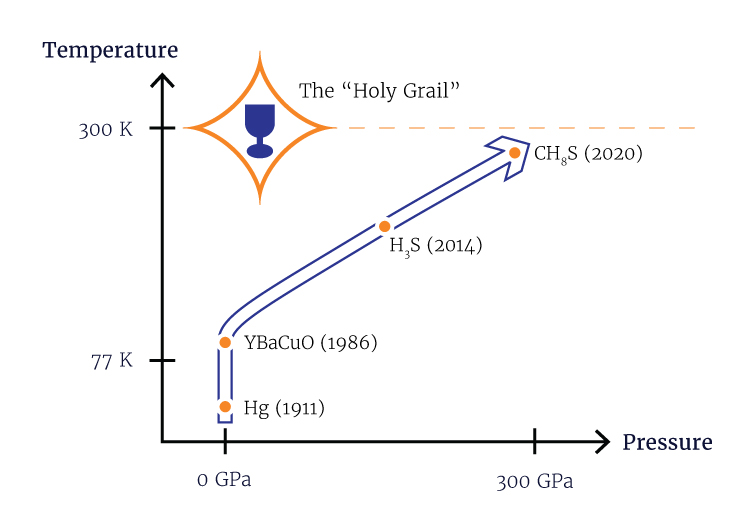
(Inside Science) -- In 2020, scientists achieved the once unthinkable -- the discovery of a material that can maintain its superconductivity at room temperature. Electrons in these materials whiz through with zero resistance -- a seemingly wonderous property with the potential to transform a host of technologies.
But there was a catch.
The superconductor, a hydrogen compound, requires pressures 2.6 million times typical atmospheric pressure to work its magic.
So, don't pop open that bottle of champagne yet, unless you want the cork to shoot right through the roof with that kind of pressure. Before we get into the century-long hunt for room temperature superconductors, let's first get a few things straight.
What is superconductivity good for?
Superconductivity was first observed in solid mercury at the super cold temperature of 4.2 K (-452 F) back in 1911. The discovery remained more or less a scientific curiosity until superconductors burst into applications later in the 20th century, mostly in the generation of magnetic fields much, much stronger than any other technique -- 10,000 times stronger than your average fridge magnet.
Without these superconductivity-enabled magnets, we wouldn't have MRI machines, or the Large Hadron Collider, which helped discover the Higgs boson in 2012. Superconducting magnets may also help us to finally achieve stable nuclear fusion one day. Talk about a butterfly effect.
But these magnets can only retain their superconductivity -- and their superstrong magnetic field -- below a certain temperature, around 10 K (roughly -440 F) for the most common material used in superconductor applications, a niobium-titanium alloy.
And it's expensive to keep things that cold.
Dry ice, which costs about $1 per pound, can take things down to 195 K (or -109 F). Liquid nitrogen, about $4 a pound, will take things to 77 K (or -321 F). To go even lower, you'll need liquid helium, which will cool things all the way down to 4.2 K (or -452 F) and can cost more than $100 a pound, depending on the supplier.
Yes. It's the same helium we fill birthday balloons with, only much, much colder.
The price of liquid helium has been fluctuating quite dramatically over the past few decades, making it difficult for research groups to budget for operational costs of the many scientific instruments with superconducting components.
So, obviously, the goal is to come up with superconductors that can operate at room temperature, because then we won't need cryogenic systems to use them. Or is it?
Raising the critical temperature is not the only important thing
"The room temperature goal is very much psychological," said Simone Di Cataldo, a physicist from the Sapienza University of Rome in Italy. "The search for something that can superconduct above the boiling point of nitrogen is much more interesting from a practical standpoint."
But we already have superconductors that work above the boiling point of nitrogen! Since 1986, numerous copper-containing compounds known as cuprates have been discovered to superconduct above 77 K. In 2006, scientists found another group of so-called high-temperature superconductors known as iron pnictides.
The problem is that currently known above-liquid-nitrogen-temperature superconductors are brittle and extremely difficult and costly to make into useful shapes, such as the coils of a superconducting magnet, said Di Cataldo.
Some of the known high temperature superconductors are also highly toxic. For example, many of the iron pnictide compounds contain arsenic.
"Just because you have a superconductor doesn't mean that you can get a lot of application out of them," said Ranga Dias, a physicist from the University of Rochester in New York.
Other material properties on the checklist for a practical superconductor include a high critical current density and a high critical field. The critical current density determines the maximum amount of electricity that can be passed through the material before superconductivity stops, while the critical field is the maximum magnetic field the material can tolerate.
So, when it comes to looking for a superconductor from a practical standpoint, where is the path with the least resistance?
A conventional path
All superconductors belong to one of two camps: conventional or unconventional. While researchers understand (more or less) how conventional superconductors work, the superconducting mechanisms dubbed unconventional cannot be adequately explained by conventional theories.
For decades after the discovery of cuprates in 1986, only unconventional superconductors had shown high-temperature superconductivity. Then in 2015, researchers observed a conventional superconductor, sulfur hydride, exhibiting superconductivity at 203 K -- although only under a tremendous amount of pressure.
"The discovery of superconductivity in sulfur hydride was a real revolution because it revealed a new type of physical system that we could play with," said Di Cataldo.
Before the discovery of hydrides, the highest critical temperature for a conventional superconductor was 39 K. Finding one that could function at much higher temperatures, even with its extremely high pressure requirement, added hope that scientists could find something that can superconduct at a desirable temperature and pressure.
Chasing down the pressure
Scientists are trying to knock down the external pressure required for hydrides to superconduct. One approach is to crank up the internal chemical pressure in these materials.
The trick is to include additional elements in the hydrogen containing crystals, with the aim of squeezing the superconducting hydrogen atoms without diluting them too much. The internal chemical pressure imposed by the extra elements can lower the external pressure required for superconductivity.
First, scientists use theory to predict combinations that may work. Then they try to make the materials in the lab and use data from the experiments to improve their models. "It's sort of a loop. We evolve as we are doing it," said Dias.
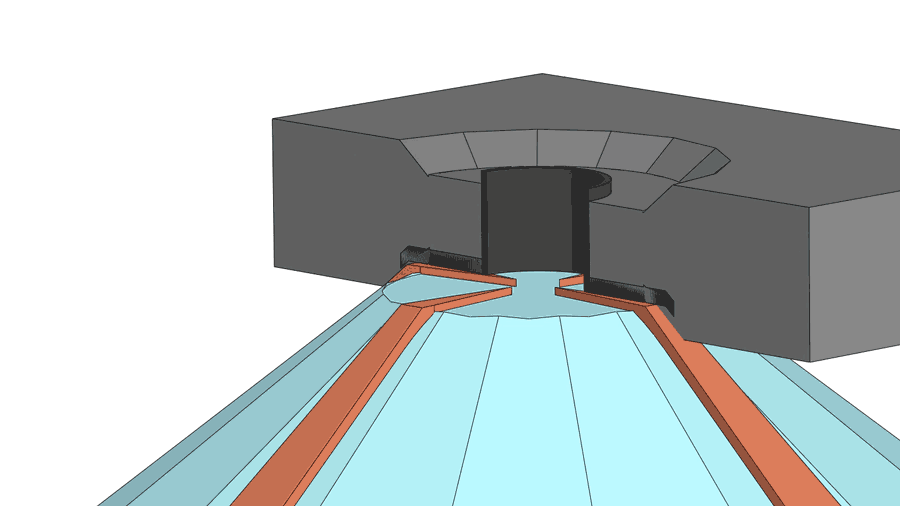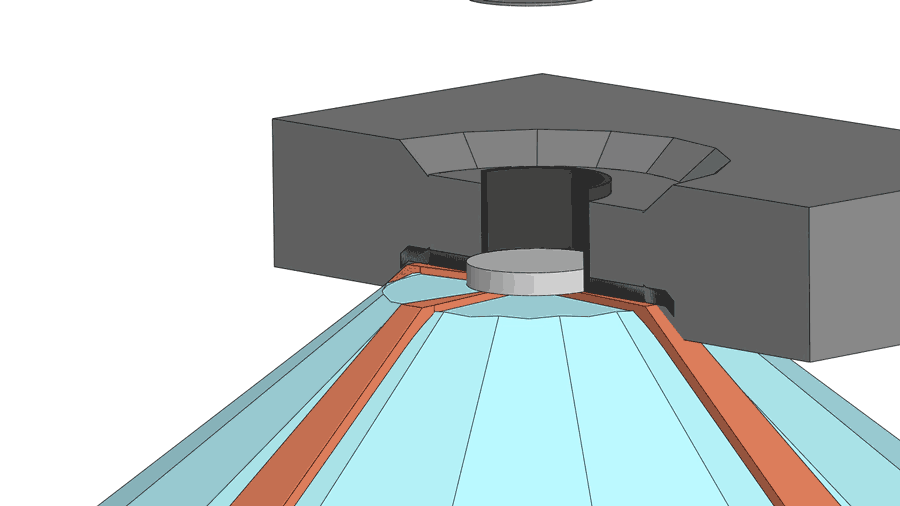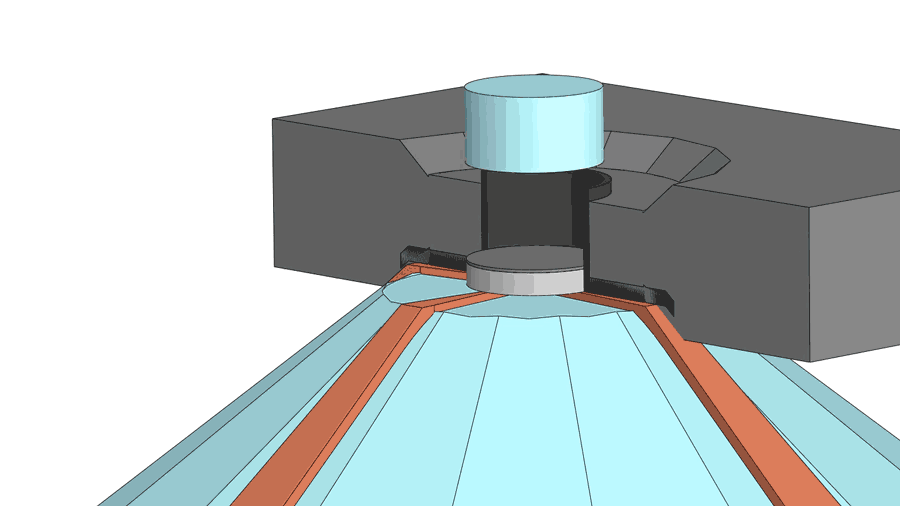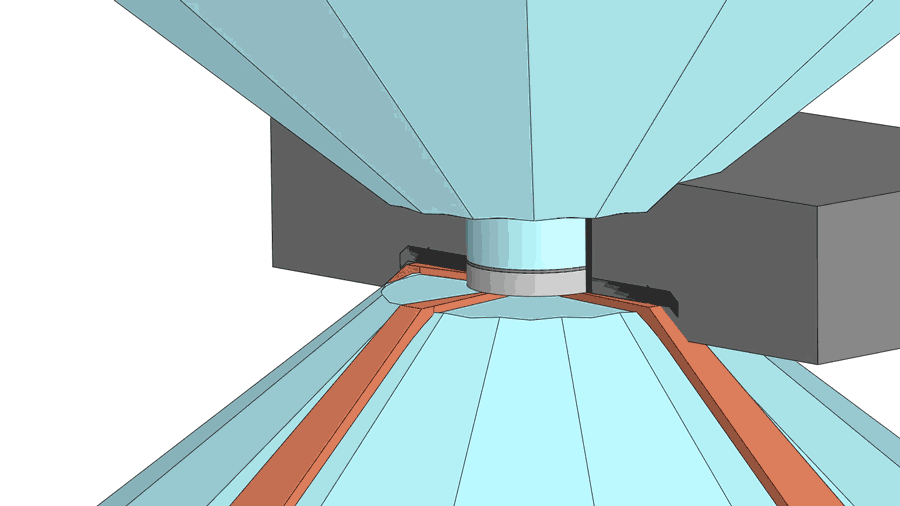
His group recently published a paper on their latest creation of yttrium superhydride, with a measured superconducting temperature of 262 K under 182 gigapascals of pressure (nearly 2 million times standard atmospheric pressure). The making of the material involves a few more chemistry tricks, including the use of a device called a diamond anvil cell to squeeze hydrogen through a sheet made from the element palladium. The sheet acts as a catalyst to help pack more hydrogen atoms into the yttrium hydride, turning it into yttrium superhydride. The sheet also serves as a shield to prevent the material from oxidizing.
Across the Atlantic Ocean, Di Cataldo and his colleagues are also looking for ways to lower the pressure requirement of hydrides, including ways to add new components to the best-performing two-component hydrides.
"Our strategy is to find a third element that can fit into the voids of a known structure, as to increase the overall packing of the atoms," said Di Cataldo.
Think of this as taking the combination of a stack of basketballs (the big atoms) packed around a collection of ping-pong balls (the hydrogen atoms), and then adding some baseballs into the mix to increase the squeeze on the ping-pong balls. According to their calculations, a hydride containing lanthanum, a large element, and boron, a small element, can be a superconductor at 40 gigapascals and 100 K, or about 400,000 times atmospheric pressure and about -280 F.
Although still massive compared to ambient pressure, the possibility of having a superconductor under 100 gigapascal would meaningfully lower the costs required to study them -- just like the seemingly arbitrary 77 K mark set by the boiling point of nitrogen.
According to Dias, a diamond anvil cell costing $3,000 to $4,000 often breaks after one or two uses for experiments above 100 gigapascal and almost always breaks for experiments higher than 180 gigapascal.
"If you are working below 100 gigapascals, then it can last months," said Dias.