Surface Bubbles Could Have Evolved into Earth's First Cells
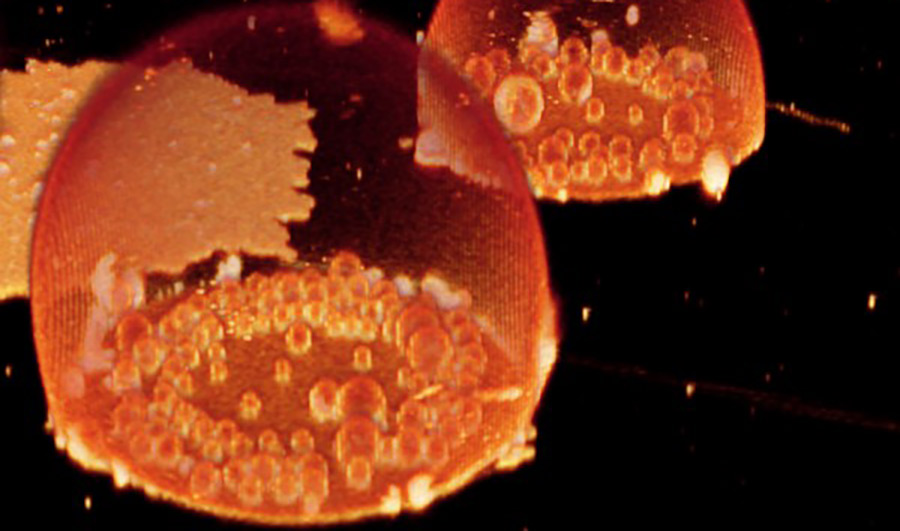
Image of “protocells” containing bubble-like compartments, which may mimic what happened 3.8 billion years ago when cells first began to form.
Image courtesy of Karolina Spustova.
(Inside Science) – Primitive "protocells" like those that evolved into the first living cells can form in bubbles on mineral surfaces that were plentiful on the early Earth, according to new research.
The researchers created artificial protocells that they believe may be similar to the protocells that may have formed on Earth about 3.8 billion years ago. The artificial protocells can absorb other small molecules by forming a barrier membrane around them -- behavior that is strikingly like that of modern living cells when they absorb cellular fuel and other essential materials while blocking off harmful substances.
And the artificial protocells also exhibit a primitive form of "division," where the outer membrane of a protocell ruptures and leaves behind several "daughter" protocells with the same capabilities.
"The role of solid surfaces in the in the context of the origin of life, specifically with respect to the development of primitive cell compartments, has been largely overlooked in the past," biochemist Irep Gözen of the University of Oslo wrote in an email. "But it appears that they can drive some amazing transformations."
Gözen leads a group of researchers whose 2020 study of the synthetic protocells was presented at the annual meeting of the Biophysical Society in February.
The researchers created droplet-shaped protocells about 50 micrometers across -- smaller than the thickness of a human hair -- by placing a solution of water and molecules called phospholipids on surfaces of aluminum oxide and silica.
Phospholipids form the membranes that separate the insides of modern cells from the outside, as well as dividing the interior of most nonbacterial cells into compartments called organelles. Most phospholipids are now created by living organisms, but they could have been formed through nonbiological mechanisms on the early Earth, or they could have been brought here by meteorites, Gözen said.
The researchers saw the liquid mixture spontaneously form into bubbles on the mineral surface. Inside those bubbles were smaller ones, which they called "daughter" bubbles. Their tests showed that the bubbles had phospholipid membranes that could encapsulate small molecules introduced from outside -- in this case, fluorescent dyes.
Most of the time, the compartmental membranes don't allow foreign molecules to get in, Gözen explained. But the artificial protocells anchored to the mineral surface periodically stretched out, then contracted.
"While stretching, they form tiny, nano-sized pores which allow the passage of these small molecules," she said. "We show that these compartments … can uptake materials from the ambient environment and maintain them there."
The artificial protocells also showed unusual behavior that mimics cell division, the process by which new living cells are created by the fission, or splitting apart, of older cells.
"In our system, after some hours, sometimes days, the original 'mother' cell disintegrates and the subcompartments become now independent 'daughter cells,'" Gözen said.
Normally, cell division is a very complicated thing, but not here: "One cannot easily split a biomembrane into two pieces. … Modern cells require a complex [biochemical] machinery for this process," she said. "We have shown that primitive cells can multiply without splitting the membrane."
The new research addresses a long-standing issue in the biochemistry of cells: How did the first cells evolve their compartments, both their outermost membrane and their internal ones, before advanced biological molecules existed to provide energy for them?
The researchers suggest that simple materials on the early Earth could have combined spontaneously to create protocells with such a compartmentalized structure, thanks to mineral surfaces that caused the primitive cells to deform and reshape: "The key player is the surfaces," Gözen said.
And if that happened, such protocells could have led to the much more complex compartmentalization mechanisms in the cells of modern forms of life. "It is fascinating to see how surfaces could substitute for the role of biological machinery that came much later in time," she said.
Biochemist Kate Adamala of the University of Minnesota, who studies synthetic cells but who was not involved in the latest research, said the study gave insights into a possible mechanism for enabling complex biochemistry on the early Earth.
"We know that key to biochemistry is keeping many different, often mutually exclusive, reactions going inside the same living cell," she said in an email. "The idea of emergence of specialized organelle-like structures [compartments] so very early in the evolution is not something we commonly discuss, but it would make perfect sense."
