
The first quantum revolution transformed science. It ultimately changed society, too. What started as physicists' quest to understand a few perplexing observations birthed a technological upheaval that has permeated the lives of billions of people on the planet. The six exhibits below showcase some of the milestones on that journey.

Let There Be Light (Particles)
1900-1905
Max Planck and Albert Einstein
Early discoveries in quantum mechanics weren't driven by a desire to shake the foundations of physics. At first, scientists simply wanted to better understand how objects emitted light of certain colors.
Nineteenth-century physicists thought of light as waves. Their formulas predicted that hypothetical objects called blackbodies, which absorb all light hitting them, should emit large amounts of ultraviolet light.
In real life, nothing actually absorbs all light that hits it. Stars come pretty close, and hot metals such as stoves and lightbulb filaments, which visibly glow, also act like blackbodies. But if lightbulbs emitted lots of ultraviolet light, like the old formulas predicted they would, they should give you a sunburn, which obviously doesn't happen.
German physicist Max Planck eventually resolved this contradiction, dubbed the "ultraviolet catastrophe," in 1901. Planck derived an equation that jibed with what scientists saw in experiments by requiring the light waves to carry energy in small packets, called "quanta."
Around the same time, Albert Einstein was grappling with what is called the photoelectric effect -- light hitting metal and knocking off electrons. Physicists thought the metal should shoot out electrons only when the brightness of the light exceeded a certain threshold. They also thought brighter light would give the liberated electrons more of a kick.
But in experiments, it was the color-- not the brightness -- of the light that determined whether electrons would fly. And it turned out brighter light knocked off more electrons, but didn't increase the individual electrons' energy. To explain this contradiction, Einstein proposed that light behaved not only like a wave, but also like a particle -- that it was made up of chunks that would later be dubbed photons. This theory also fit with Planck's idea that light could carry only specific amounts of energy.
A Portrait of An Atom
1909-1913
Ernest Rutherford and Niels Bohr
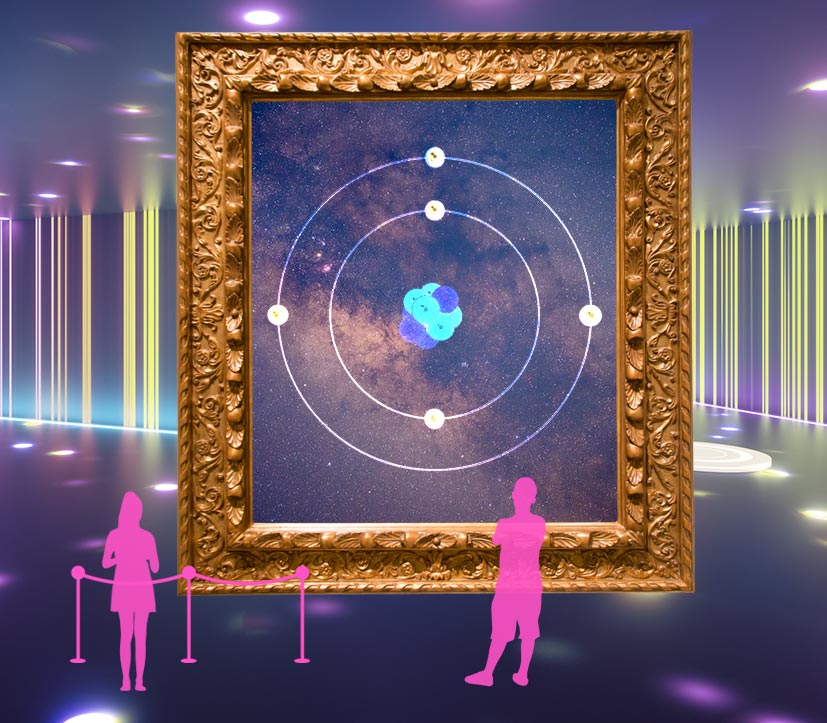
As physicists revamped their view of light, matter also got a closer look. In 1904, British physicist J.J. Thomson cooked up what he called a "plum pudding" model of the atom, with small, negatively charged electrons as the bits of fruit stuck in a larger, positively charged cake.
But a few years later, Thomson's former student Ernest Rutherford spoiled the dessert analogy with his famous "gold foil" experiment. He discovered that some of the positively charged particles he and his colleagues shot at a sheet of gold foil bounced back, almost "as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you," Rutherford wrote. Rutherford reasoned that the atom's positive charge must be densely packed in its center, in what we now call the nucleus, and the electrons were orbiting it through mostly empty space.
But the new model had its own problem: What was preventing the center from pulling the electrons inward and destroying the atom? Danish physicist Niels Bohr rode to the rescue. Inspired by Planck and Einstein's idea of discrete packets of energy for light, he proposed that electrons could also have only specific energies, and thus would orbit the nucleus from specific distances, kind of like planets orbiting a star.
Later scientists realized the paths of electrons could not be completely measured. (Read more about this development in "The Uncertainty of the Particle" exhibit.) Bohr's planetary model was supplanted by the idea of electron orbitals, which describe the probabilities of electrons being in certain locations. The electron orbitals theory still prevails today.

Particles Do the Wave
1924-1927
Louis de Broglie, Clinton Davisson and Lester Germer
Bohr's model of the atom was given a boost by the work of French physics student Louis de Broglie.
De Broglie started by simply taking the idea of light behaving like a particle and turning it on its head: What if particles (such as electrons in an atom) could act like light?
A defining property of light is its wavelength -- the distance from one peak to the next. In 1924, de Broglie developed an equation for the wavelength -- of a particle.
For everyday objects like apples and ants and apricots, de Broglie's equation predicted wavelengths so incredibly miniscule as to be unnoticeable even with the most sophisticated equipment. But for tiny particles such as electrons, his formula had significant implications. For one thing, it explained Bohr's electron orbits: The orbits Bohr predicted were also the distances from the nucleus where the electrons' wavelengths wouldn't overlap with themselves.
A few years later, two American physicists, Clinton Davisson and Lester Germer, performed an experiment whose results supported de Broglie. When they fired a beam of electrons at a nickel crystal, the electrons scattered in the way predicted by de Broglie's formula.
So-called double-slit experiments performed later also support de Broglie's idea. In a double-slit experiment, electrons are shot at a barrier with two narrow holes in it. Observed one at a time, the electrons seem to behave like particles, passing through the slits and landing as dots of light on a screen behind the barrier. But as the dots accumulate, a mysterious pattern of vertical bands appears on the screen. The electrons completely avoid some parts of the screen and collect in other parts -- the same pattern that would be produced by waves passing through the slits.

The Uncertainty of the Particle
1926-1927
Erwin Schrödinger, Max Born, Werner Heisenberg, Albert Einstein and Niels Bohr
De Broglie's equation predicted that tiny particles such as electrons would have relatively large wavelengths compared to their size. (For example, the wavelength of an electron moving at around 6 million meters per second would be roughly the same size as an atom.) But what does that mean for understanding how the particles act?
Physicists described the behavior of traditional waves, such as those of light and vibrating strings, with something called a wave equation. Inspired in part by de Broglie's idea that matter could have wavelike properties, as well as by some work by Einstein in 1924 on a quantum theory of gases, Austrian physicist Erwin Schrödinger published a version of a wave equation for particles in 1926. But at first, it wasn't clear what the equation signified. German physicist Max Born helped make sense of it later that year when he argued that it described the probabilities of a particle having certain properties.
Born's interpretation seemed to imply that the universe has some inherent uncertainty baked into it. Unlike classical mechanics, which allows you to predict exactly where an object moving in a particular direction at a particular speed will end up, quantum mechanics seemed to suggest that one could describe only the probabilities a particle might end up in certain places. In 1927, German physicist Werner Heisenberg expanded upon this idea of uncertainty when he proposed that the more you know about an electron's velocity, the less you know about its position, and vice versa.
At first, Heisenberg thought this uncertainty arose because measuring these properties interfered with the particles. But Niels Bohr believed that Heisenberg's equation described a more fundamental feature of the universe -- that particles don't actually have specific positions and velocities at the same time and don't have precise values for these properties until they are measured.
Bohr and Einstein debated these ideas at the 1927 Solvay conference on physics in Brussels, where leading physicists from around the world gathered to discuss electrons and photons. Bohr argued that at most, only the probability of a future state can be predicted. This interpretation clashed with Einstein's (and probably your) view of reality -- that objects must exist and have defined qualities at all times.
Einstein believed that the theory of quantum mechanics must be incomplete. The idea of fundamental uncertainty also made Schrödinger himself uneasy. But over time, Bohr's interpretation became the prevailing one.
A note about the cats: The cats roaming Quantum 2.0-ville are a nod to a famous thought experiment by Schrödinger, who thought a probabilistic interpretation of reality was a ridiculous proposition. To illustrate his point, he described the following scenario: Imagine a cat trapped in a box with a vial of poisonous gas and a radioactive atom. The box also contains a detector that, if the atom decays, will break open the bottle of poison, freeing the gas and killing the cat. (To be clear -- Schrödinger never attempted this experiment.)
By the probabilistic interpretation of quantum mechanics, before the atom is measured, it is in a superposition of decayed and not decayed. So, Schrödinger argued, before you look in the box, the bottle of poison is in a superposition of broken and intact, and the cat is in a superposition of dead and alive. Schrödinger pointed out that the idea of a cat being both dead and alive at the same time until you looked at it was absurd, so he thought this probabilistic view of quantum mechanics couldn't be correct.
Now, if this experiment were performed in real life, the cat would never be in a superposition of dead and alive. A human doesn't need to be present to determine the atom's state -- if the atom decays, the detector will sense it, and the atom will no longer be in a superposition. While we don't see a cat in a superposition of life and death, physicists have managed to get molecules up to a few thousand atoms in size into a quantum superposition. Physicists still don't all agree on an interpretation of superpositions.

The Fall of Local Realism
1935-2015
John Stewart Bell, John Clauser and others
Bohr and Einstein's tussle over the meaning of quantum uncertainty continued into the 1930s. In 1935, Einstein devised a thought experiment with fellow physicists Boris Podolsky and Nathan Rosen, published in what's referred to as the EPR paper, to show how seemingly absurd Bohr's interpretation of quantum mechanics was.
The argument relied on the idea that when two or more particles interact, their properties could become linked, or correlated. (Schrödinger later described such particles as entangled.) Correlated properties exist in everyday life too. For example, the handedness of one glove is correlated with its partner. If you know one is right-handed, then you also know the other must be left-handed.
But imagine if the gloves were particles. If Bohr's interpretation of quantum mechanics is true, then neither glove would have a defined handedness until one is measured. At that point, the handedness of the other glove would also instantly snap into place, no matter how far away it is. Einstein thought this "spooky action at a distance" shouldn't be possible, and thus quantum mechanics shouldn't be considered a complete description of reality. (Instead of glove handedness, the EPR paper uses the position and momentum of entangled particles as examples of correlated properties.)
The thought experiment makes more sense if the handedness of the gloves (or the position and momentum of the particles) were in fact predetermined, but we just couldn't tell what they were until we measured them. In the 1960s, Irish physicist John Stewart Bell proposed an experiment that, in theory, could reveal whether or not particles carried such predetermined properties (sometimes called hidden variables).
John Clauser carried out the first version of this experiment in the early 1970s. Later experiments by others eliminated some loopholes present in early versions. Each time, the results showed that particles don't seem to have predetermined properties, striking a blow to the intuitive interpretation of the EPR experiment. Some physicists think these results rule out a commonsense view of the world called local realism, which says that particles have defined properties and disconnected particles cannot influence each other faster than the speed of light.
A Whole New Quantum World
20th century
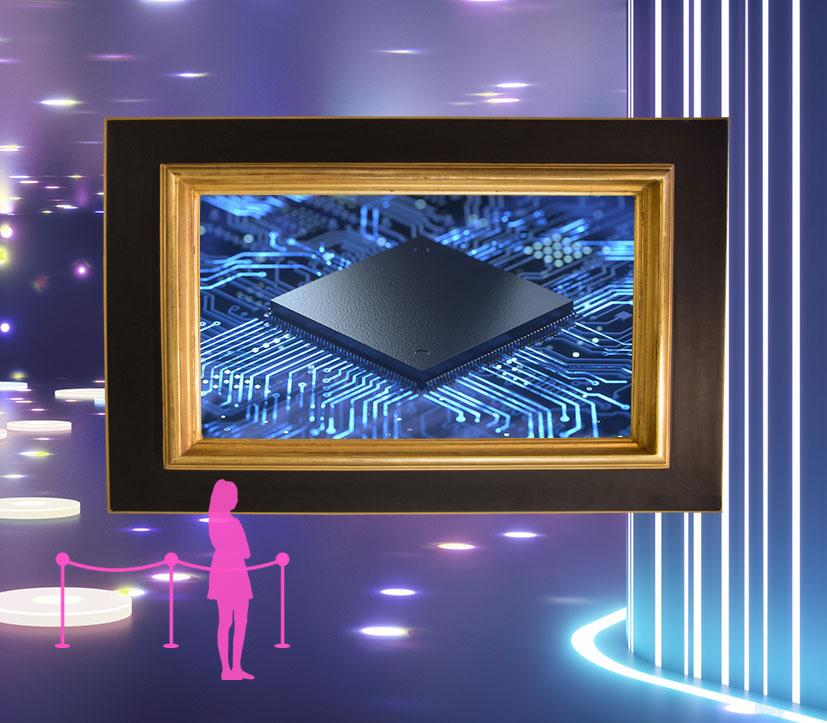
Discovering the counterintuitive behavior of some of the universe's smallest things didn't just change the way physicists think. It also led to the development of modern technologies that changed the world.
Once scientists better grasped how atoms and light behave, they were able to more effectively manipulate materials to produce useful gadgets. The invention of computer chips, atomic clocks and lasers all depended on an understanding of quantum concepts, such as the idea that electrons could have only specific amounts of energy. These three technologies alone have transformed the way we live, putting computers in the pockets of billions of people; guiding our cars, ships and planes; and changing the way we check out groceries, perform surgeries, make phone calls, and even do science.
The profound and ubiquitous consequences of the first quantum revolution can be found all around us, from the moment we wake up to the moment we shut our eyes at night. Who knows what quantum revolution number two has in store?
The New Quantum Frontier is brought to you by Inside Science, an editorially-independent news service of the American Institute of Physics.





